

We know that time is money. Our decades of experience has given us the knowledge that the best remedy for minimizing costly overruns and/or missing important project milestones is to start any project with the cornerstone of user-centered design… by conducting early design research known as Contextual Inquiry. Our Human Factors team excels at conducting Contextual Inquiry to uncover critical insights early in a project. This "show me, don’t tell me" approach reveals vital details about users’ daily workflows, behaviors, work settings, pain points, and unmet needs that other methods often miss. These insights lay the groundwork for establishing project milestones within Design Controls, align teams on common design and development goals, and lead to intuitive, safe, and effective products that minimize use errors, enhance usability, and ensure a smoother pathway to regulatory approval.

Task Analysis
The Stratus Design HFE team understands that the Task Analysis is an essential tool in the HFE toolkit. It provides a detailed ledger of the intended user interactions with your device or system. The advantage of employing this tool is to provide each member of the design and development team a holistic and granular view of which task(s) will be performed by which user(s). The outcome is tighter team alignment through shared understanding of the intended use and minimizing costly misalignments and misunderstandings that waste time, effort, and money.
The Stratus Design HFE team can help you create and complete your Task Analysis and ensure team member alignment which will guide design improvements and supporting regulatory compliance ultimately leading to better patient outcomes and market success. Here's more information about the advantages and key benefits of completing a Task Analysis:
Use-related Risk Analysis
At Stratus Design, we prioritize our commitment and partnership to ensure the safety, effectiveness, and user-friendliness of medical devices through our comprehensive approach to the Use-related Risk Analysis (URRA). This systematic approach is integral to our support of your development process, helping to identify potential use-related risks and hazards associated with device use and coming up with viable design-related risk control measures.
Here's how we leverage URRA to enhance our approach to your device development project:
Analysis of Know Use-related Problems
We conduct thorough analyses of known use-related problems to ensure the safety and effectiveness of your medical device in development. This process aligns with Regulatory Guidance and recognized Standards, which require manufacturers to identify past use-related issues as a critical input to the Use-related Risk Analysis (URRA). Our analysis focuses on the “Gold Standard” predicate and/or marketed products with similar user interfaces, interactions, and intended uses, drawing on adverse event databases like MAUDE, customer complaints systems, and insights from product trainers as well as the users, themselves. The outcome of our analysis is a comprehensive list of identified use errors, along with existing or necessary risk control measures. These findings can then be converted into specific user interface requirements for the new device, ensuring robust safety measures are in place. By learning from past experiences, we help our partner clients develop safer, more user-friendly medical devices that meet Regulatory Standards and enhance user satisfaction and adoption.
Analysis of Adverse Events
At Stratus Design, we approach the analysis of adverse events with a dedication to improving patient safety and enhancing the overall user experience of medical devices. Our approach involves reviewing each event from the perspective of the human, the suitability of the device's user interface, as well as considering other performance-shaping factors. Our HFE specialists have the proven expertise to thoroughly investigate how design flaws may have induced adverse events to provide actionable insights to improve device safety and usability. Through these analyses, Stratus Design helps clients implement effective risk control measures and enhance the safety and usability of their medical devices.
Human Performance and Ergonomics Analyses
Our team of experienced HFE specialists excel in performing analyses targeted to evaluate the ergonomics of device’s design and how well it supports human capabilities and limitations. By including anthropometric analyses, we help partner clients develop devices that are not only compliant with industry Standards but also ensure that designs fit the physical dimensions and capabilities of intended end users by enhancing ergonomics, safety, and accessibility.
Some examples include:
Market Analyses using HFE
The quality of information expected and required in Regulatory submissions for marketing approvals has become greater, more detailed since Regulatory bodies, like the FDA, EMA, and the NMPA (a.k.a., the Chinese FDA), have released guidance documents detailing their expectations. Stratus Design has the expertise to help navigate this space and conduct Gap Analyses of HFE documentation within Design Controls in advance of the final submission. We ensure that the documentation meets all regulatory requirements and industry Standards as well as preparations for the Notified Bodies, like TÜV and CE. By identifying any missing or inadequate documentation, a Gap Analysis facilitates a smoother regulatory review process, thereby reducing the risk of delays or rejections during submission.
Threshold Analysis
Stratus Design specializes in conducting thorough Threshold Analyses which is crucial for identifying differences between a proposed medical device and an existing predicate or reference product. Our team examines all aspects of the user interface and interactions, comparing them to existing products to identify minor and significant design differences. We leverage this analysis to justify the applicability of existing HFE data, ensuring regulatory compliance and streamlining the development process. Through our expertise, we help partner clients control project risks, enhance device usability, and facilitate regulatory review processes.
State of the Art Analysis
The European Union Medical Device Regulation (EU MDR) requires manufacturers to account for the current state of the art when designing and manufacturing medical devices. The State of the Art reflects what is generally accepted as best practice in technology and medicine, and, according to ISO 14971:2019, is also an accepted and defined term. Stratus Design’s HFE specialists perform a thorough State of the Art analysis by comparing new product designs to competitor devices, standards, and published data recognized as state-of-the-art. This analysis ensures the new device conforms to the safety principles of current state of the art devices and assesses the acceptability of the new device's risks.
Human Factors/Usability Evaluation
Improving Usability and Controlling Use-related Risks
Stratus Design excels in conducting both formative evaluations and human factors validation testing. Our team uses a systematic approach to identify and mitigate use-related risks through iterative testing and user feedback. This ensures that the device's user interface is intuitive, safe, and effective. By conducting thorough evaluations, we help our clients achieve a high level of usability, which is crucial for market success and user satisfaction.
Providing Traceability for Regulatory Compliance
We understand the importance of traceability in design controls. Our detailed documentation process aligns with regulatory expectations, including those of the FDA, NMPA, and International Standards like IEC 62366. We also have the expertise to provide comprehensive records of all HFE activities, from initial risk assessments to final validation testing, ensuring that every step is traceable and compliant with regulatory requirements.
Formative Evaluation
At Stratus Design we know that time and effort is money. Saving both means identifying issues early to reduce the likelihood of costly design changes later in the development process and ensure a clearer path to regulatory approval. Conducting formative evaluations early and continuously exposes design strengths and opportunities for improvement, allowing for the identification of potential use-related risks and the implementation of associated risk control measures.
Formative evaluations range in level of detail and exploration as well as cost, time, and effort. Stratus Design’s HFE team partners with clients to help determine which type of formative evaluation is most appropriate based on influencing variables like project phase, design maturity, availability of time, resources, and/or funding.
Here are examples of the options for Formative Evaluations:
Human Factors Validation / Summative Testing
At Stratus Design, we have years of experience conducting comprehensive Human Factors Validation (Summative) Testing to demonstrate that a medical device can be used safely and effectively by the intended users without serious use errors or problems. This type of testing verifies that the final design of the device's user interface supports safe and effective use under expected conditions.
Our team’s expertise includes:
By integrating rigorous observational and qualitative data analysis, we identify root causes of use errors and can partner with our clients to implement necessary design modifications, if necessary, and prepare the final reports required as a part of the final submission package to Regulators.
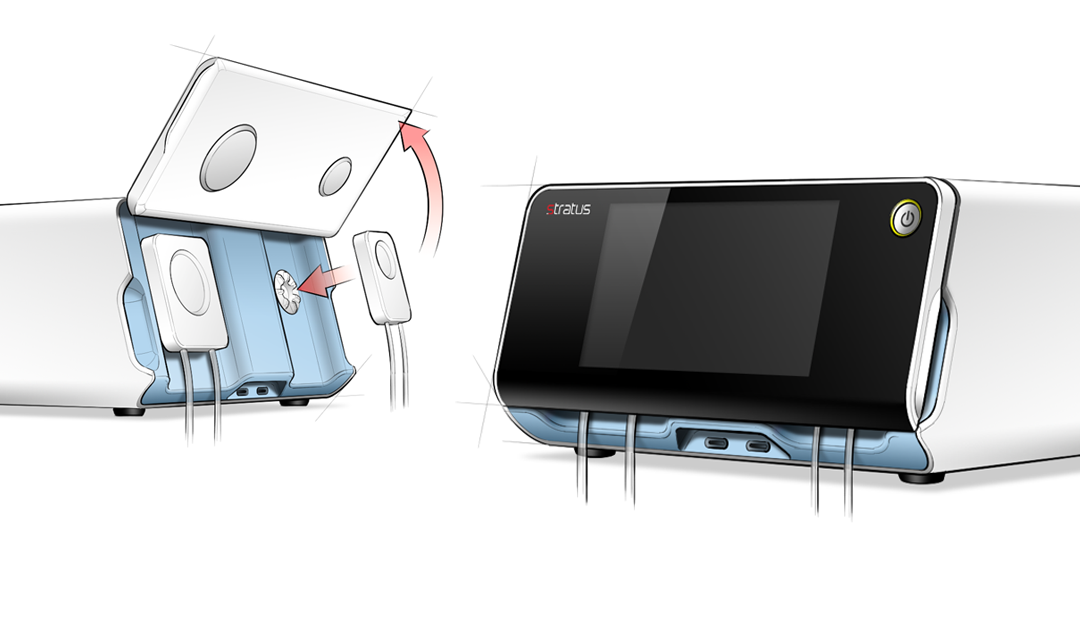
Stratus Design's industrial design philosophy centers on creating distinctive products that are not only visually appealing but also perfectly integrated into the larger user ecosystem and aligned with your brand's identity. Our designs are tailored to encourage the correct usage through intuitive interfaces and ergonomic features, ensuring they are suitable for their intended environments.
From conceptual sketches to detailed CAD models, our team ensures every design is optimized for manufacturability without compromising on style or functionality.
We combine analytical rigor with creative insight to leverage early discovery insights and deliver products that enhance user experiences and succeed in the marketplace.
Discovery Research and Market Opportunity Identification
Effective ID research encompasses a range of activities aimed at understanding user needs, market trends, technological advancements, and manufacturing processes to inform and enhance product development.
Cross-disciplinary research fosters innovation, integrates diverse perspectives, and ensures that products meet technical requirements, market demands, and user expectations. It involves collaboration with engineers, marketers, psychologists, and other experts.
Workflow / Journey Mapping and Research Data Visualization
Workflow and Journey Mapping visualizations provide clear representations of processes, workflows, procedures and device use across the continuum of care. This clarity helps teams and stakeholders understand how tasks are interconnected, who is responsible for each step, and the sequence of activities involved. They illustrate relevant data to help identify bottlenecks, redundancies, and inefficiencies in processes, to streamline workflows, optimize resource allocation, and improve overall productivity.
These powerful tools improve organizational efficiency, decision-making, and understanding across diverse disciplines. They transform abstract concepts and raw data into actionable insights, driving design inputs and requirements.
Concept Sketching and Ideation
Early concept sketch exploration is a fundamental design activity that enables us to illustrate, communicate and evaluate many ideas quickly and intuitively. It serves as a medium to explore different design possibilities, iterate on concepts, and brainstorm creative solutions.
Sketches facilitate communication between designers, stakeholders, and clients by conveying design concepts in a visual format, enabling clearer and more effective communication of design intents and visions. They play a crucial role in translating abstract ideas into tangible design solutions and driving successful product outcomes.
Conceptual CAD Development
Conceptual CAD (Computer-Aided Design) modeling offers several advantages in Industrial Design, complementing traditional sketching by providing a more realistic representation, helping designers and stakeholders better understand the spatial relationships, proportions, and overall form of the product early in the design process. Configuration models ensure proper internal component packaging, overall dimensions and ergonomics, allowing stakeholders to evaluate initial concepts in a realistic context.
UX / UI Design
Conceptual UI (User Interface) design refers to the process of creating visual representations and interactions for physical and digital interfaces, such as software applications, websites, and interactive devices. Our process focuses on the needs and behaviors of users, ensuring interfaces are intuitive, safe, aligned with user expectations, and enhancing the overall experience.
Combining ID and UI design activities is essential for creating products with a coherent design language, where the physical aspects of the product align seamlessly with its digital interface, promoting consistent branding.
Parametric CAD Modeling / DFM
DFM (Design for Manufacture) CAD modeling is a proactive approach that integrates manufacturing considerations into the design process, ensuring that products are not only functional and aesthetically pleasing but also optimized for efficient and cost-effective production.
Our designers use the same CAD software as our mechanical engineers to ensure a seamless and efficient collaboration, and to preserve the carefully established aesthetics and design intent throughout the development process.
Brand Identity and Development
Industrial Design is instrumental in shaping brand identity through consistent visual elements, materials, and forms across product lines. A well-executed design can convey brand values and personality, helping to strengthen brand recognition and loyalty among consumers.
CMF Design
CMF (Color, Material & Finish) design is a strategic aspect of product development that influences aesthetics, user experience, brand identity, market appeal, and sustainability. By integrating thoughtful CMF strategies, brands can create products that not only meet functional requirements but also inspire emotional connections and drive success in competitive global markets.
Photo-realistic Product Renderings
These high-quality visualizations provide accurate depictions of product designs, offering CMF and branding options for evaluation and iterative refinement, while reducing costs and accelerating time-to-market. They provide visually accurate Preference Study stimuli and enhance presentations and collaborative discussions, fostering alignment among stakeholders and ensuring design consistency throughout the product lifecycle. They can also serve as powerful marketing tools by effectively communicating product features and design intent, while enhancing brand perception.
Instructional Design and Development
Instructional Design development is a crucial activity that ensures products are safely and effectively used by consumers. By providing accurate information, clear instructions, and regulatory compliance, manufacturers enhance use safety, satisfaction, and trust in their products while meeting legal and regulatory requirements in global markets.
Appropriate graphic illustrations and diagrams complement textual instructions in IFUs. They help clarify complex procedures, demonstrate proper usage techniques, and illustrate safety precautions effectively.
Labeling and Packaging Design
Effective packaging design integrates aesthetics, functionality, sustainability, and brand communication to create a compelling consumer experience while meeting practical requirements for product protection and market competitiveness. It plays a pivotal role in influencing consumer perceptions and purchase decisions, making it a critical component of successful product marketing strategies.
3D printing, Prototype Design and Assembly
Physical models are used throughout the design process for conceptual and ergonomic evaluations, ranging from quick and simple foam core models to 3D printed CAD models and works-like-feels-like prototypes.
To support use evaluations, we design, manufacture and assemble prototypes as is appropriate per study parameters. These can range from neutral looking, de-branded works-like prototypes to functional appearance models.
GUI Prototyping
Clickable prototypes support usability testing, offering accelerated development timelines and risk mitigation. They enhance collaboration between stakeholders, validate design decisions, and ultimately contribute to the creation of intuitive, user-friendly, and successful digital interfaces.
VR Study Development
Virtual Reality (VR) is invaluable for assessing design choices before physical prototypes are being built. It lets participants experience the product in real size and in a controlled environment to evaluate space constraints, visual sight lines, accessibility, and overall functionality. It allows designers to experience the world through the users' eyes and illustrates ergonomic challenges for various anthropometric groups from their perspectives.
VR evaluations can accelerate the development cycle by allowing rapid iteration and testing of multiple design concepts in a time and cost efficient manner before committing to physical prototypes, particularly for large scale projects.

Engineering is where ideas turn into reality. As your project evolves from insightful observations to a fully formed concept, our engineering team is here to guide it every step of the way. We push the limits of what is possible to bring your design vision to life. From defining initial requirements and determining internal space needs to discussing manufacturing considerations, exploring new technologies and providing detailed fabrication documentation - the engineering team is here to ensure that the design intent is preserved in the final product.
At Stratus, we pride ourselves on being problem-solvers rather than obstacles. When requirements clash or we push the boundaries of a design, our team carefully evaluates the trade-offs for each option and presents solutions that overcome potential challenges to meet your goals.
Specification Gathering and Requirements Documentation
Defining success at the beginning helps create a distant point that the development team can work towards. Engineers have a seat at this table next to many other stakeholders to make sure that requirements are fully captured and communicated, are reasonable in scope and don’t contradict one another. In our mission to carry conceptual design into practical application, early involvement helps smooth the process by reducing communication errors and rework.
Systems Engineering
Early-stage systems engineering helps align teams from the outset. A proactive approach helps identify inter-dependencies and define roles and responsibilities. The goal is lower risk by illuminating a streamlined path to a product. Early coordination requires less total work.
Design Work
Design work is the heart of Stratus - we all participate in guiding an idea into a product. Engineering collaborates heavily with ID in the design phases to help identify and work through problems.
Detailed CAD
We take models from many sources – Conceptual models from ID, Client CAD, purchased parts, etc – and combine them into a well-organized structure that is made from parts and assemblies that are ready for fabrication. We can mesh with your team to work in a way they understand to ensure that the files are transferable and maintainable in the long term.
Manufacturing Documentation
As a crucial part of design transfer, we collect and document all the details required to reliably manufacture a product. We can communicate directly with your supplier to keep your project accurate and on track. We create fabrication drawings that document how you want your product made in a way that fits with your documentation system, assign part numbers, build BOMs and work directly in your PDM system if you’d like.
Preserving Design Intent
The work done in the design phase provides an original vision for the product - a blend of aesthetics, functionality, and user experience that must remain intact as it’s being readied for manufacturing. Maintaining design intent safeguards the usability, ergonomics and style of the product to preserve the magic created in the design phase. By keeping engineering and design teams aligned we help make products that are not just functional, but also meaningful.
Physical Model Creation
We create models to learn more about aspects of a design – especially those intended for human interaction. Below are several kinds of models our engineering team when the situations demands:
Motion Analysis
We can design and check specific ranges of motion for components. This could mean a complex linkage synthesis, knowing speeds and accelerations at important points along the path. It could mean checking for interferences as parts articulate or open and running through modes of operation. Or it could be helping you evaluate a model in virtual reality to make the concepts more accessible to those who might have input.
Stress/Strength Analysis
We use FEA to evaluate parts and look for problem areas. We can compare old and new designs to show strength gains, or provide an initial validation that a design is suited for it’s purpose. Depending on client needs, the engineers will either generate a formal report or use the results iteratively during the design process.
Design for Manufacturing
DFM is a large topic, but we’re here to help you where you need. We can help with process selection, material selection, selecting off-the-shelf parts, recommending vendors and more. Leverage our experience and networking to expand your network or try a new process.
Troubleshooting
Why doesn’t this work? Well… let’s have a look